Amnio AMP-MP
AmnioAMP-MP™ is a chorion-free, dual- layer dehydrated human amnion membrane allograft derived from the amniotic lining of the placenta. The protective properties of amnion make it an ideal barrier to protect soft tissue from the surrounding environment. AmnioAMP-MPT™ is processed through minimally manipulated techniques. This type of processing retains the qualities of the native extracellular matrix (ECM). AmnioAMP-MP™ can be applied in any orientation and quickly hydrates in situ and naturally stays in place eliminating application placement limitations.
Safety and Versatility
- Amniotic tissue is recovered from healthy mothers who have undergone full-term delivery
- AmnioAMP-MP™ is processed in accordance with FDA regulations and AATB standards
- Amnioti tissue has been used for over 100 years with well-documented clinical success
- Requires no up-front preparation
- Ambient temperature storage
- Can be applied on either side of the graft
- E-Beam sterilization provides a sterility assurance level (SAL) of 10-6
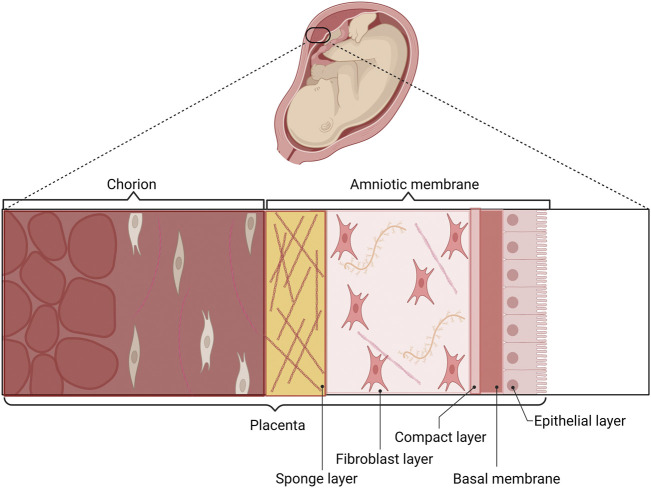

Placental membranes: all-natural wound protection.
AmnioAMP-MP™ provides a protective extracellular matrix barrier for use in covering wounds and soft tissue. The product has been used as a barrier to protect diabetic ulcers, venous ulcers, pressure ulcers, burns, and other dermal ulcerations that include wounds with exposed vital structures such as tendons, muscles, or bones.
| PRODUCT CODE | NAME | SIZE (L x W, centimeters) |
| CG1100 | AMNIO AMP-MP | 2X2 cm |
| CG1101 | AMNIO AMP-MP | 2X4 cm |
| CG1104 | AMNIO AMP-MP | 4X4 cm |
| CG1105 | AMNIO AMP-MP | 4X6 cm |
| CG1106 | AMNIO AMP-MP | 4X8 cm |
AmnioAMP-MP™ process retains the qualities of the native ECM. Growth factors and other mediators: (not inclusive of all)
- EGF
- bFGF
- KGF
- PDGF
- VEGF
- TGF-beta 1 and -beta 3
- HGF
- TIMP/MMP
- NGAL
Proteins found in AmnioAMP-MPT™ include:
- Collagen I, III, IV, V and VII
- Elastin
- Fibronectin
- Laminin
| Resources | Brochure Download |
|---|---|
| AmnioAMP-MP™ | Click Here |